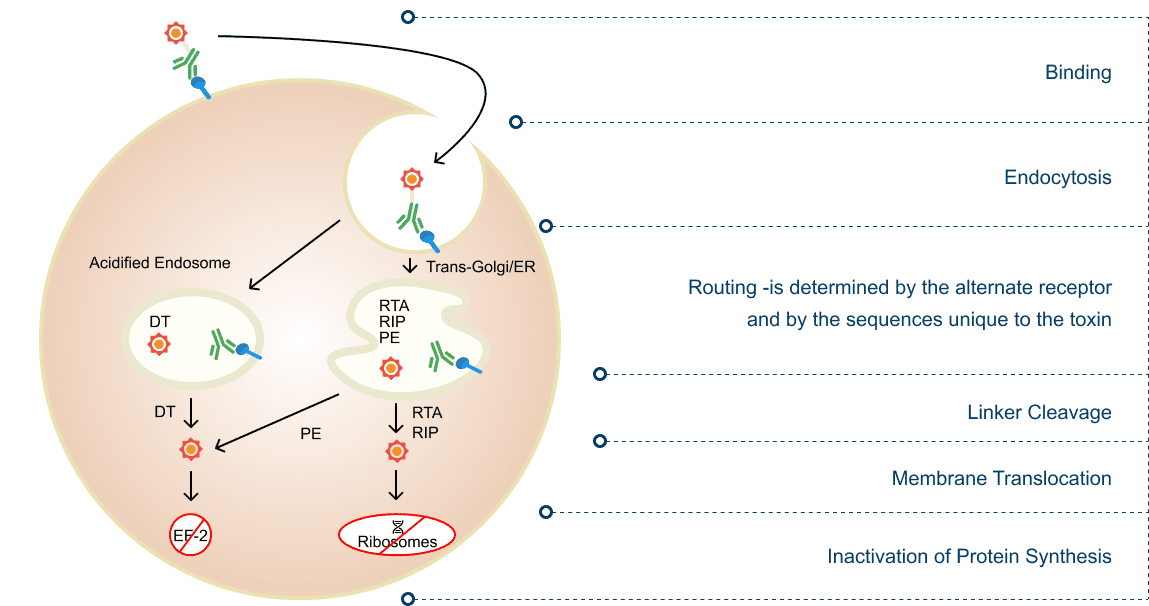
A bivalent fusion protein: two antibody-binding domains targeting T cells attached to a truncated diphtheria toxin. VG712 binds to CD3 antigens on T cells, endocytosis delivers the toxin inside T cells and leads to cell death

1Unlike monoclonal antibodies, scFVs have no Fc binding sites and therefore do not trigger lethal cytokine storm
2Truncated toxin reduces toxicity to non-CD3 cells by 1000-fold
3Half-life of 40 minutes in human body decreases risk of infection and other complications
1Eliminates all T cell subsets since CD3 is expressed on almost all mature T cells
2Bivalency increases binding affinity to provide potent biologic activity
3Potency allows short treatment cycle: 4-day treatment is sufficient to deplete up to 99%+ of all T cells

Dr. David Neville, a pioneer in immunotoxin and team invented Resimmune
Dr. Huaizhong Hu optimized molecule design for large scale production
Developed efficient and reliable production platform
Obtained global patent protection

Licensed Resimmune from NIH for development
Completed P1 clinical trial in CTCL; gained FDA Fast Track status and Orphan Drug Designation
Collaboration with U. Louisville to initiate research for anti-PD-1 combination therapy
Collaboration with U. Colorado and completed POC study for prevention of kidney transplant rejection

Virogenbio was formed to license VG712 global development and commercialization rights from Angimmune
CMC development is complete, and drug products are ready for large-scale clinical trials
FDA clearance received for a pivotal Phase II clinical trial in CTCL, and Phase I trials in PTCL and aGvHD
Kidney transplant Phase I/II trial is ready for IND submission
Research and development collaboration agreement established with Moffitt Cancer Center
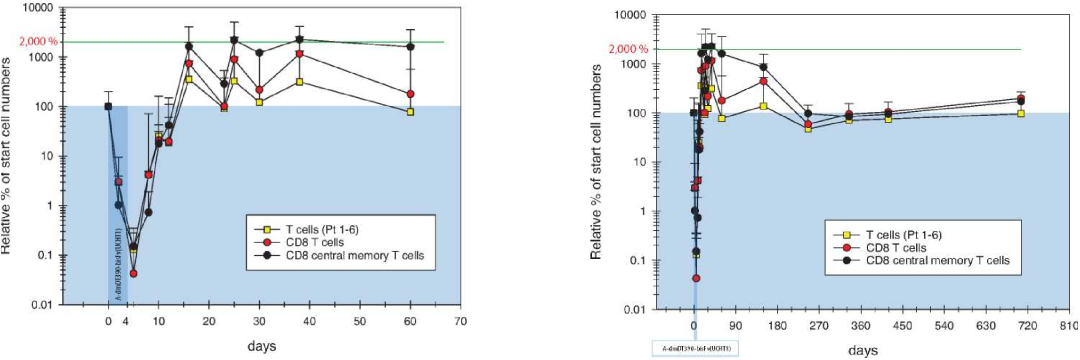
VG712 clears up to 99%+ of all T cell subtypes
T cell depletion is transient: after the 4-day treatment, substantially complete T cell depletion induces immediate and robust homeostatic proliferation
Proliferation peaks at up to 20 times pre-treatment levels and begins to normalize around day 90

CTCL n=24, 19 patients stages 1b-2b, 5 patients stages 3-4
Overall Complete Response Rate (CRR) 17%, higher than available treatments
Response particularly robust among 12 intermediate stage patients (as measured by mSWAT lesion size score): 33% CRR, with 3 patients achieving complete remission for a duration of 6 years+ and 1 patient 4 years+